The Purox® Process"The Pure Oxygen Process"
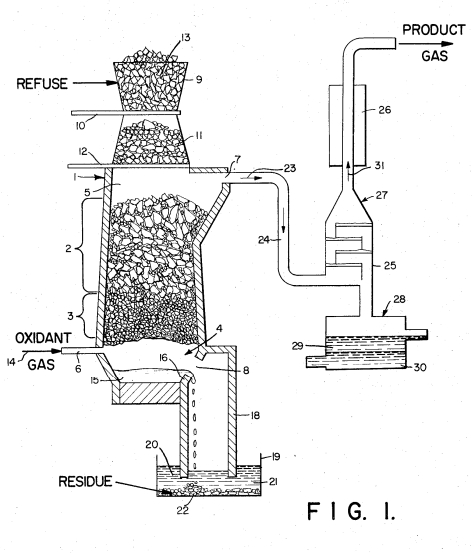
An Initial LookThe Purox process was developed by Union Carbide in the early 1970's. The underlying patent was awarded to John Anderson in 1973; here's a diagram of the process as described in Patent 3,729,298:
The goal of the process was to be able to convert Municipal Solid Waste (MSW) into a useful heating gas and inert cinder. In the height of the energy crisis of the early 1970's, serious consideration was given to creating a Purox facility that would serve the Seattle area. The resolution of the energy crisis resulted in a significant drop in interest in all forms of alternative energy including this one.
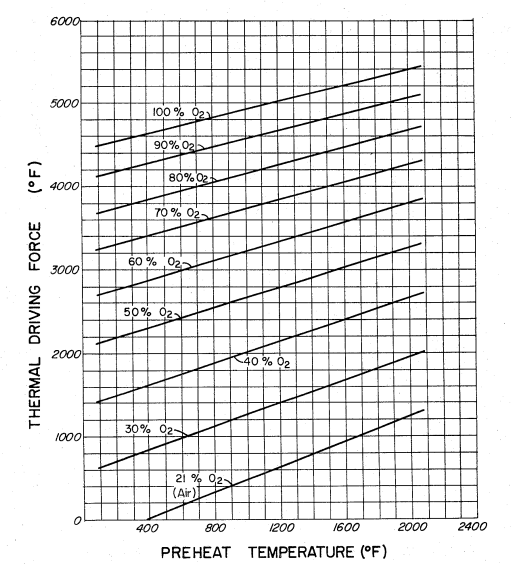
Some Background DetailsAfter drying, the first step in the gasification of biomass is pyrolysis, i.e. the breaking down of organic compounds in an oxygen-deprived containment. As a result, the utility of using pure oxygen is somewhat counter-intuitive. When biomass gets hot enough, it will break down into a tarry form of producer gas; the book Farenheit 451 was named after the temperature at which paper will start breaking down into combustible gases. When biomass starts to burn, what's actually burning is the combustible gases that the heated material gives off, not the actual material itself. All biomass contains some amount of water; even "dry" firewood contains between five and ten percent water depending on the humidity. So, the first step involves driving off that associated water, something which is generally complete at around 220°F. Then it takes even more heat to start to break down the woody components such as lignin into combustible gas. In most forms of gasification, the heat needed to make this happen comes from burning a portion of the gas given off by the heated biomass. That heat drives off more combustible gas which in turn burns and accelerates the pyrolysis until nothing is left but charcoal. Combustion is a form of oxidation, and most gasifiers use air as the oxidizer. Air is cheap enough, in the sense that it's available for free, but from a thermal-efficiency perspective it's expensive in that only one fifth of air is oxygen with nitrogen forming the remaining four fifths. As a result, gasifiers that use air as the oxidizing gas have to heat up five times as much air as would be needed if they were using pure oxygen as the oxidizer. Since it's the heat given off by the oxidation of the biomass that drives the reaction, the key concern is the ability of the incoming gas to drive the reaction to temperatures that are hot enough to complete the conversion of the biomass into wood gas. Here's a graph that shows how the ability of the oxidizer to thermally drive the reaction goes up as the percentage of oxygen goes up.
As the graph shows, using pure oxygen as the oxidizer completely transform the reaction's thermodynamics. A reactor operating at a substantially higher temperature is desirable for a number of reasons. For example, a hotter reactor produces a gas mixture that has a higher heat value. Assuming there's enough heat available to drive the endothermic reaction, a molecule of carbon dioxide (a non-flammable gas) will react with the solid char (carbon) to produce two molecules of carbon monoxide (a combustible gas). CO2 + C + heat => 2CO
Another simultaneous reaction that increases the heat value of the gas produced involves the reaction of any moisture originally contained in the biomass with hot carbon.H2O + C + heat => H2 + CO
The heat value of the gas can be further increased by injecting pure oxygen and steam into any remaining charcoal using a technique known as "auto thermal steam reformation." This is a thermodynamically balanced process in which the thermal energy (heat) given off when charcoal combines with pure oxygen is used to drive the endothermic reactions described above.Increasing the heat value of the gas is important for a community-based energy system. While an air-fed gasifier readily yields a gas that is capable of operating a village bakery or laundry, an oxygen-enriched gasifier can produce a gas with an energy content that will enable a resilient village to melt glass, fire pottery, kiln cement and cast iron. In this way, the B2M Project has the potential to enable a community to move beyond meeting its basic energy needs to being able to create value-added products, work that will provide sustainable jobs and merchantable goods. Sourcing the OxygenWhen Union Carbide introduced the Purox process in the mid-1970's, pure oxygen was produced by the fractional distillation of liquid air, a process which is energy intensive and technologically challenging. The requirement for large quantities of cheap oxygen limited the application of the Purox technology to large, industrial applications. In the 1990's, the development of Pressure Swing Adsorption ("PSA") systems changed the playing field. A PSA unit uses a molecular sieve to separate air into two streams: nitrogen, and a mix that's 95% oxygen and 5% argon. While the oxidizer generated by the PSA is not pure oxygen, it's close. An air-driven gasifier generally produces a gas that is half nitrogen; eliminating that inert portion by using pure oxygen results in a gas that has twice the heating value. That also moves us a significant step closer to the production of a gas that's more suitable as an input for the Fischer-Tropsch, LPMeOH and Mahajan fuel-condensation reactions. Note: Purox® is a registered trademark of Union Carbide, Inc. |